We’re Just a Call Away!
Need help? Give us a call for a free consultation with one of our specialists.
Mobility Scooters, Sunshine Coast




SKU: ZAS1000
Couldn't load pickup availability
At Kineticare, we're committed to offering not only the highest quality mobility solutions but also the best value for our clients. That's why we proudly introduce our Price Beat Guarantee. If you find a lower price from an identical local competitor, we promise to not just match it, but beat it. We're committed to providing you with premium products at unbeatable prices, ensuring you can choose Kineticare with confidence for all your mobility needs.
Experience the Kineticare difference - superior products, unparalleled service, and our Price Beat Guarantee. Contact us today to learn more!
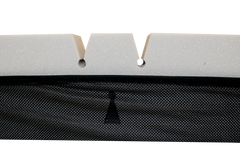
DUAL THERAPY – STATIC IMMERSION & ACTIVE AIR ALTERNATION
The globally recognised indication of a support surface’s effectiveness in pressure injury (PI) prevention has been through its ability to either reduce the peak strain/stress values in tissues to constant low pressure (CLP) such as a pressure redistribution support surfaces (Refer to High Care Mattress Range) or by decreasing the continuous exposure time of tissues to the sustained strain/stress state such as by repositioning or alternating air support surface. Advances in the technology of support surfaces are now moving toward a combination of both the aforementioned indicators of support surface effectiveness.
Support surfaces known as a ‘Hybrid’ are one such example of combining the technologies of CLP and AS through the use of both powered Air cells in conjunction with Foam configured wither as a layer of foam (either simple or castellated foam) at the patient interface (Known as‘ foam above cell’), or as powered air cells around the foam inserts, which effectively provides a layer of alternating air cells above the foam.
Debate amongst healthcare professionals also questions the efficacy of including CLP into an AS, where the question is asked, will there be sufficient offload to allow for the body’s natural blood flow and responsive measures in preventing pressure injuries such as reactive hyperaemia. Studies have shown that smaller pressure amplitudes, such as those seen with a Hybrid AS, generally provide sufficient pressure relief to maintain tissue viability, whereas when the pressure amplitudes are larger like the 100/0 traditional AS, then there is a tendency toward compromised tissue viability even in able-bodied subjects.
A 100/0 AS will provide such high peak pressures (even though there is a 100% offload) that the surface can provide more harm than good to a user. Moreover, studies into the speed of pressure injury have shown that the incidence of high pressures particularly against bony prominences can result in “massive apoptotic cell death within Minutes”.
The unique configuration of the Zephair incorporating static immersion and alternating therapy, also know as hybrid, is designed to reduce overall peak pressures across the patient interface of the support surface.
The major difference between a traditional AS and a Hybrid AS includes the amount of ‘offload’ achieved. For example, in a 1:2 alternating air system, for every cell ‘A’, 100% of the body’s mass is supported on 50% of the cells, with every second cell achieving full ‘offload’ to allow for blood flow and relief of pressure – A system known as 100-0 or ‘High Amplitude’ System. A hybrid system, in contrast, will not achieve the full offload, but will always have ‘pressure’ of some form on both the inflated and deflated cells, due to the nature of foam – its tendency to ‘spring back’ into position. A hybrid system may achieve an offloading result closer to 80-20 with 80 percent of the body supported on 50% of cells and 20% of the body supported on the remaining cells.
One such product utilising the advancement of hybrid technology is the Forté Healthcare Zephair Hybrid Powered Alternating Air Support Surface, operating with the FA configuration. The FA configuration stands out in the industry as a comfort-enhancing product, mainly due to the foam reducing or eliminating the feel of a ‘plastic-like’ air cell.
Often postulated between health care professionals is the belief that for an alternating air pressure mattress to operate effectively, placing objects on the surface that may obstruct the movement of air between the cells must be avoided. The result of which means that FA surfaces such as the Zephair are overlooked due the foam being perceived as a ‘hindrance’ to clinical function and acting as a barrier between the air cells and the user.
The industry good practice statement is in fact referring to objects which do not provide pressure redistributing or pressure relieving properties. Medical continence care products such as a waterproof sheet or pad are the intention of the statement due to the mechanical nature of such products. Waterproof sheets, for example are often constructed from PVC/Vinyl, which as a rigid material, provides little to no stretch. A non-stretch material like PVC will prevent a user from sinking into the mattress and act much as a ‘hammock’, suspending the user ‘above’ the mattress rather than ‘in’ the mattress, thus presenting the industry statement is indeed a relevant and worthy consideration.
However, in the case of a FA hybrid mattress such as the Zephair, foam is a pressure redistributing substance, allowing for deflection and stretch around bony prominences. The foam used above the cells acts to provide more comfort to the user through two forms:
1) Reducing the ‘amplitude’ between inflated and deflated cells
2) Creating a more plush surface
Comfort = Compliance
When pain is relieved by the support surface:
a. The individual is more sensitive to discomfort and pain which may indicate concentrated pressure – a warning sign that there is a risk of pressure injury formation
b. Better sleep = less fatigue = more stamina = better physical and mental endurance for client and caregivers
c. Reduces need for pain and sleep medication – improves cognitive state, less strenuous care regimens
Sizes and Availability
ZAS1000 - Stocked - Single
ZASL1000 - Stocked - Long Single
ZAKS1000 - Stocked - King Single
ZAKSL1000 - Stocked - Long King Single
ZAEW1000 - Made to Order - Extra Wide
ZAEWL1000 - Made to Order - Long Extra Wide
ZAD1000 - Made to Order - Double
ZADL1000 - Made to Order - Long Double
ZAQ1000 - Made to Order - Queen
ZAQL1000 - Made to Order - Long Queen
ZAK1000 - Made to Order - King
ZAKL1000 - Made to Order - Long King
ZASQ1000 - Made to Order - Split Queen
ZASQL1000 - Made to Order - Long Split Queen
As a registered NDIS provider, Kineticare recognises the significance of collaborating with plan managers and NDIS participants to deliver optimal support and care.
Kineticare is committed to providing high-quality, cost-effective, and accessible back care and mobility products to NDIS participants throughout Australia. We take pride in being a reliable partner for plan managers and the broader disability community. For more information or to request a quote, please click the link above or click here.
Thanks for subscribing!
This email has been registered!
| Product | SKU | Rating | Description | Collection | Availability | Product Type | Other Details |
|---|
Need help? Give us a call for a free consultation with one of our specialists.